Introduction
The rationale for periodontal therapy is the elimination of periodontal disease, restoration of periodontal tissues to healthy functional state and subsequent maintenance of the restored tissues.[1] Periodontitis affected root surfaces are hypermineralized, pathologically altered and are contaminated by bacterial endotoxin which inhibit growth and vitality of fibroblasts in vitro and may prevent new connective tissue attachment (Hanes PJ et al 1991).[2]
Thus, for regeneration to occur, disinfection and modification of the contaminated root surface in order to restore its biocompatibility and to favour the attachment of regenerated periodontal structures becomes the necessity.[3] Although meticulous root planing by hand instrumentation or by ultrasonic scalers have been advocated, root surface will inevitably be covered by smear layer acts as a barrier for connective tissue attachment to the root surface.
Therefore, to enhance the effectiveness of root planing, chemical root conditioning was introduced in order to detoxify, decontaminate and demineralize the root surface, thereby removing the smear layer and exposing the collagenous matrix of dentin and cementum.[4] A variety of agents have been used that include hydrochloric acid, citric acid ,ethylene diamine tetra acetic acid ,tetracycline ,stannous fluoride, fibronectin, cohnn’s factor IV, sodiumdeoxycholate, growth factors, minocycline hydrochloride, phosphoric acid etc.[5]
Citric acid (pH 1) has been proved to be an efficient root conditioning agent. It has been shown to remove smear layer, demineralizes the planed root surfaces, elutes bacterial endotoxins from pathologically altered cementum surfaces, prevent epithelial migration along the denuded root surface and enhance attachment either by connective tissue in growth or by splicing of newly formed collagen to the exposed dentinal fibrils.[6]
Recently, the use of calcium chelators such as EDTA with neutral pH has been shown to hold considerable promise as root conditioning agent. Studies have shown that EDTA selectively removes hydroxyapatite, leaving most of collagenous matrix intact, preserves the adjacent tissue vitality, promotes early cell and tissue colonization by providing a more biocompatible surface for cell and has been reported to give favourable results with respect to less flap failure and more connective tissue attachment.[7]
Materials and Methods
Patient Selection
Patients in the age group of 30-50 years (both male & female) suffering from generalized chronic periodontitis were selected amongst those visiting the Department of Periodontology and Implantology, Govt. Dental College and Hospital, Patiala.
Selection Criteria
1) Cooperative patients, showing acceptable oral hygiene during phase 1 therapy.
2) Patients not having any systemic problem that contraindicate periodontal surgery.
3) Absence of attrition, abrasion or erosion.
4) Absence of internal or external root resorption.
5) Absence of any root caries or restoration on root surfaces of experimental teeth.
6) Teeth with furcation involvement will not be selected.
Study Design
Fifteen patients having two almost identical bony defects with pocket depth ranging from 4-8 mm, one on either side of same arch were selected. Surface of the selected tooth having maximum pocket depth was considered for the study and were randomly divided into two sites.
Site I: Citric acid (pH 1) was applied as root conditioning agent.
Site II: EDTA (pH 7-neutral) was applied as root conditioning agent.
Pre-surgical Management
All subjects received a full diagnostic work up that included intraoral periapical radiograph according to area of interest and clinical examination to record plaque score, pocket depth and clinical attachment level with occlusal stent as a guide. Subjects were given oral hygiene instructions. Thorough scaling and root planing was performed and, occlusal adjustments were done if necessary to relieve traumatic occlusion.
Preparation of Citric Acid (pH 1.0) Freshly prepared saturated solution of citric acid at pH 1 was used as root conditioning agent on one side. Citric acid solution was prepared by adding citric acid in anhydrous form into distilled water at room temperature under continuous mixing until solution became saturated. pH 1 was attained using pH meter. Solution was then filtered using Whatman filter paper # 1.
Ethylene diamine tetra acetic acid (EDTA pH 7-neutral)EDTA liquid at pH 7 was used as root conditioning agent on other side of same arch.
Disclosing Solution – 0.075% solution of basic fuchsin. Before scaling, 15 ml of solution was given to each subject to rinse the mouth for 20 seconds followed by two rinses with plain water (6gms of basic fuchsin was dissolved in 100 ml of 95% ethyl alcohol. The concentrated dye alcohol solution was further diluted by adding 7,900 ml of distilled water to get the required 0.075% of dye solution).
Stent-Preparation
Occlusal stents for positioning the measuring probe were fabricated with cold-cure acrylic resin on a cast model of each patient obtained from an alginate impression. The occlusal stent was trimmed flat on the bottom edge, and vertical locating groove was made on the facial interproximal aspect with bur for the proper guidance and orientation of the periodontal probe. Using the groove as a guide, the periodontal probe was inserted into the pocket and clinical measurements were obtained.
Recording of Clinical Parameters
Following clinical parameters were obtained immediately before surgery (day 0) and subsequently at the end of 6 weeks.
1) Plaque Index (Quigley-Hein and Elliot) using disclosing solution (0.075% basic fuchsin).
2) Probing pocket depth (Using Williams calibrated periodontal probe)
3) Clinical attachment level (Using Williams calibrated periodontal probe and customized acrylic occlusal stent).
Surgical Management
A complete and comprehensive medical and dental history examination of all the subjects was taken. Subjects were given an explanation of the study purpose and a signed consent from the patient was obtained.
Area was anaesthetized with 2% xylocaine (1:200,000 adrenaline) and crevicular incision was given from the base of the pocket to the crest of the bone. The muco-periosteal flap was reflected and thorough scaling and root planing was done. The entire area was irrigated with normal saline solution. On experimental tooth in one segment, citric acid solution was applied passively with cotton pellet for 3 minutes and then the area was irrigated with normal saline. During application of citric acid, the exposed surfaces of the flaps were protected with saline moistened gauze. Pellets were changed every 30 seconds to avoid dilution of acid. On experimental tooth of other side of same arch, EDTA solution was applied with cotton pellet passively for 3 minutes and then the area was irrigated. Pellets were changed every 30 seconds to avoid dilution of acid. Flaps were adapted back to their original position and interrupted suturing was done using non-resorbable silk suture (Mersilk 3-0).The surgical area was then covered with Coe-Pak.
Post-surgical Management
Following medicines were prescribed orally Antibiotic: Cap symbiotic (Amoxycillin 500mg + Lactic acid bacillus)- 1 tds for 5 days ,Analgesic and anti-inflammatory drug: Tab Brufen 400 (Ibuprofen 400 mg)- 1 tds for 3 days and Capsule B-complex with vitamin C (Becozyme C-forte)- 1 OD for 5 days
Seven days after, surgical dressings and sutures were removed and the site was cleansed.
Recording and Recall Visits
Recording of all clinical parameters was carried on day 0 (baseline) and subsequently at the end of 6 weeks.
Statistical Analysis
Statistical Analysis was performed using a statistical package, SPSS windows version 15 by applying mean values using analysis of variance (ANOVA) and student-t test.
Results
It was observed that both the materials were well tolerated by all the patients with no adverse reaction and infection during the course of study. In both sites, a significant reduction in PI, PD and CAL gain was observed at the end of 6 weeks as seen in Table 1. On comparison between site I and site II, reduction in PI and PD and CAL gain was more in site II but the difference was statistically non significant (p>0.05) as seen in Table 2, 3 and 4.
 | Table 1 : Mean Plaque Scores, Mean Pocket Depth Scores (mm), Mean Scores Of Clinical Attachment Level (mm) Of Site I And Site Ii At Different Time Intervals.
 |
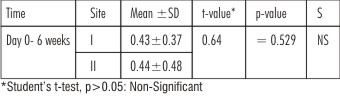 | Table 2 : Showing Comparison Of Reduction In Mean Plaque Scores Of Site I And Site II At Different Time Intervals
 |
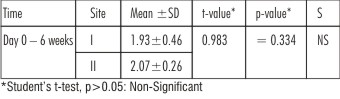 | Table 3 : Showing Comparison Of Reduction In Mean Periodontal Pocket Depth Scores (in mm) Of Site I And Site II At Different Time Intervals
 |
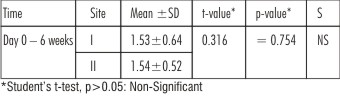 | Table 4 : Showing Comparison Of Gain In Clinical Attachment (in mm) Of Site I And Site II Level At Different Time Intervals
 |
Discussion
The nature of the periodontally exposed roots has been identified as one major factor influencing periodontal regeneration. Cementum surfaces exposed by periodontitis are pathologically altered, hypermineralized and contaminated with periodontal pathogens and endotoxins.[4] Such surfaces are not biocompatible with adjacent periodontal cells, the proliferation of which is pivotal for periodontal wound healing (Polson AM et al 1982).[8]
Root surface conditioning has been suggested using a variety of agents in order to detoxify, decontaminate and demineralize the root surface.[9] Detoxification of root surface helps in removing the cementum bound bacterial endotoxins which have been shown to inhibit the growth and viability of fibroblasts in vitro and may prevent new connective tissue attachment. Surface demineralization of the radicular dentin removes the smear layer, uncovers and widens the orifices of dentinal tubules (Polson AM et al. 1984)[10] and exposes the dentinal collagen matrix (Selvig KA et al. 1981).[11] This collagen matrix is thought to provide a substrate which supports the chemotaxis, migration and attachment of cells involved in wound healing and formation of new connective tissue attachment.
A variety of root conditioning agents have been used to enhance the new attachment on root surface that include hydrochloric acid, citric acid, ethylene diamine tetraacetic acid, tetracyclines, stannous fluoride, fibronectin, cohnn’s factor IV, sodiumdeoxycholate, growth factors, minocycline hydrochloride, phosphoric acid etc.
In this study, in site I, citric acid solution (pH 1) and in site II, EDTA (pH 7) was used as root conditioning agent along with periodontal flap surgery.
Citric acid has been shown to alter the root surface characteristics of treated root surfaces (Garrett JS et al. 1978),[12] has antibacterial property (Daly CG et al 1982),[13] induce cementogenesis, promote collagen splicing (Garrett S et al. 1978),[12] augment fibronectin-fibrin-collagen binding thereby inhibits the epithelial apical migration (Polson AM & Proye MP 1982)[8] and enhance fibroblast chemotaxis, migration and attachment (Boyko GA et al. 1980).[14]
It has been shown that EDTA acting at neutral pH is as effective as low pH etchants with respect to smear layer removal and superior in exposing root surface collagen (Blomlof J & Lindskog S 1995).[7] EDTA is the only agent which exclusively exerts its demineralizing effect through chelating divalent cations at neutral pH. Studies have shown that chelating agent (EDTA) working at neutral pH appears preferable with respect to preserving the integrity of exposed collagen fibers, early cell colonization, and periodontal wound healing and it also preserves adjacent tissue vitality (Blomlof J et al 1995,[7] 1996,[15] 2000[16] )
In the present study, passive application was preferred over burnishing technique as the latter may itself form smear layer which may partially or completely obliterate the dentinal tubule openings (Wen CR et al. 1992).[6]
Plaque Scores
In both site I (Citric acid) and site II (EDTA ), reduction in mean plaque score at the end of 6 weeks from baseline (day 0) was statistically significant (p<0.05) as seen in Table 1. The results are in accordance with the observations made by (Caffesse RG et al. 1987)[17] and (Mayfield L et al. 1998).[4]
Comparison of mean plaque score reduction between site I and site II was statistically non significant (p>0.05) as seen in Table 2. Similar results were recorded by (Blomlof L et al. 2000).[16]
In site I and site II, reduction in supragingival plaque score could be attributed to good oral hygiene practiced by the patients during the entire study period (Jeong et al. 1994).[18]
Periodontal Probing Depth
In site I (Citric acid ) and site II (EDTA), reduction in mean pocket depth at the end of 6 weeks from baseline (day 0) was statistically significant (p<0.05) as seen in Table 1. This is in accordance with the studies conducted by(Blomlof L et al. 2000)[16], (Mayfield L et al .1998)[4] ,(Blomlof PS et al .1996)[15]
In the present study, reduction in pocket depth in both the sites (site I & site II) is due to resolution of gingival inflammation by scaling and root planing and reduction that occurs in healing by tissue shrinkage and attachment gain after application of root conditioning agents along with periodontal flap surgery(Blomlof J et al. 1995 [7] ,2000 [16] & Blomlof PS et al. 1996) [15]
On comparison,although, mean pocket depth reduction was more in site II, but it was statistically non significant (p>0.05) as seen in Table 3.
Clinical Attachment Level
In site I (Citric acid) andsite II (EDTA), Gain in mean clinical attachment level at the end of 6 weeks from baseline (day 0) was statistically significant (p<0.05) as seen in Table 1. This is in accordance with the studies conducted by, (Mayfield L et al. 1998)[4] ,(Blomlof L et al. 2000)[16] & (Parasnis et al. 2006)[19].
On comparison,gain in mean clinical attachment level was more in site II, but it was statistically non significant (p>0.05) as seen in Table 4. Similar results were recorded by (Blomlof J et al. 1995)[7]
There was more reduction in pocket depth and gain in mean clinical attachment level in site II which may be due to ability of EDTA to selectively expose collagen fibers than etching with citric acid acting at low pH, which may in turn act as a chemo attractant for periodontal fibroblasts. EDTA also preserves the integrity of exposed collagen fibers, adjacent tissue vitality, there by promoting early cell colonization and periodontal wound healing (Blomlof & Lindskog 1995).[7]
Moreover, gain in clinical attachment level might also be due to improved health of surrounding soft tissues after periodontal flap surgery, which offers increased resistance to probe penetration (Paranis et al. 2006).[19]
Within the limits of the study, both citric acid and ethylene diamine tetra acetic acid (EDTA) proved to be beneficial in removing smear layer and facilitating periodontal wound healing and new connective tissue attachment when used as an adjunct to periodontal flap surgery.
Difference between the results of present study and those of other studies may be related to variations in patient selection, patient compliance, time and mode of application of the demineralizing agent or a combination of these variables. Hence, additional studies both in vivo and in vitro of these variables with better standardization and larger sample size are needed.
Conclusion
Within the limits of the study, it can be concluded that both citric acid and ethylene diamine tetra acetic acid (EDTA) are beneficial in removing smear layer and facilitating periodontal wound healing and new connective tissue attachment when used as an adjunct to periodontal flap surgery.
Although results of citric acid (pH1) and EDTA (pH7-neutral) were comparable and difference was statistically non- significant but EDTA acting at neutral pH seems to be preferable to citric acid because it selectively removes hydroxyapatite leaving most of collagenous matrix intact and has no necrotizing effect on surrounding periodontal tissues.
References
1. Smith BA, Smith JS, Caffesse RG, Nasjleti CE, Lopatin DE, Kowalski CJ. Effect of citric acid and various concentrations of fibronectin on healing following periodontal flap surgery in dogs. J Periodontal 1986; 58: 667-673.
2. Hanes P, Polson A and Fredrick T. Citric acid treatment of periodontitis affected cementum. A scanning electron microscopic study. J Clin Periodontal 1991; 18: 567-575.
3. Pant V, Dixit J, Agrawal AK, Seth PK, Pant AB. Behaviour of human periodontal ligament cells on CO2 laser irradiated dentinal root surfaces: an in vitro study. J Periodontal Res 2004; 39: 373-379.
4. Mayfield L, Soderholm G, Norderyd O, Attstrom R. Root conditioning using EDTA gel as an adjunct to surgical therapy for the treatment of intraosseous periodontal defects. J Clin Periodontol 1998; 25: 707-714.
5. Sterret JD, Bankey T, Murphy HJ. Dentin demineralization. The effects of citric acid concentration and application time. J Clin Periodontal 1993; 20: 366-370.
6. Wen CR, Cafesse RG, Morrison EC, Nasjletti CE, Parikh UK. In vitro effects of citric acid application techniques on dentin surfaces. J Periodontal 1992; 63: 883-889.
7. Blomlof J, Lindskog S. Periodontal tissue vitality after different etching modalities. J Clin Periodontal 1995; 22: 464-468.
8. Polson AM, Proye MP. Effect of root surface alterations on periodontal healing. II. Citric acid treatment of the denuded root surfaces. J Clin Periodontal 1982; 9: 441-454.
9. Melcher A. On the repair potential of periodontal tissues. J Periodontal 1976; 47: 256-260.
10. Polson AM, Frederick GT, Ladenheim S & Hanes PJ. The production of a root surface smear layer by instrumentation and its removal by citric acid. J Periodontal 1984; 55: 443-446.
11. Selvig KA, Ririe CM, Nilveus R & Egelberg J. Fine structure of new connective tissue attachment following acid treatment of experimental furcation pockets in dogs. J Perio Research 1981; 16: 123-129.
12. Garrett JS, Crigger M, Egelberg J. Effects of citric acid on diseased root surfaces. J Periodontal Res. 1978; 13: 155-163.
13. Daly CG. Anti-bacterial effect of citric acid treatment of periodontally diseased root surfaces in vitro. Journal of Clinical Periodontology 1982; 9: 386-392.
14. Boyko GA, Brunett DM, Melcher AH. Cell attachment to demineralized root surfaces in vitro. J Periodontal Res 1980; 15: 297-303.
15. Blomlof J PS, Blomlof LB, Lindskog SF. Smear removal and collagen exposure after non-surgical root planing followed by etching with an EDTA gel preparation. J Periodontol 1996; 67: 841-845.
16. Blomlof L, Jansson BA, Blomlof J, Lindskog S. A clinical study of root surface conditioning with an EDTA gel. II. Surgical Periodontal Treatment. Int. J Periodontics Restorative Dent. 2000; 20: 567-573.
17. Caffesse RG, Alapach SR, Morrison EC, Burgett FG. Lateral sliding flaps with and without citric acid. IJPRD 1987; 61: 43-56.
18. Jeong S, Han S, Lee S et al. Effects of tetracycline containing gel and a mixture of tetracycline and citric acid containing gel on non surgical periodontal therapy. J Periodontol 1994; 65: 840-847.
19. Parasnis AO, Tsiklakis K and Tatakis DN. EDTA gel root conditioning: lack of effect on clinical and radiographic outcomes of intrabony defect treatment with enamel matrix derivative. J Periodontal 2006; 77: 103-110.
|